Long Acting Ionically Paired Pamoate-based Suspension of Lurasidone: An exploration of Size Effects on in vitro Dissolution and in vivo Pharmacokinetic Behaviors

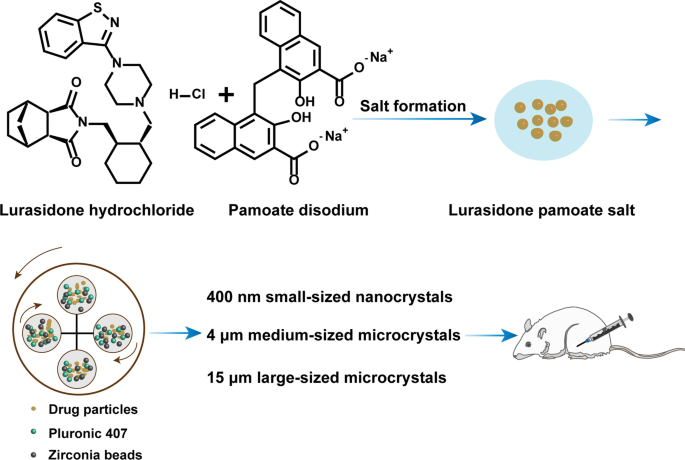
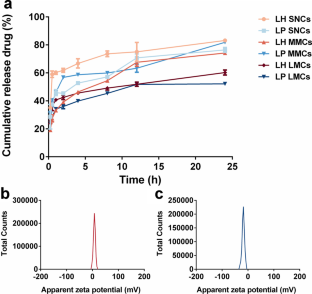
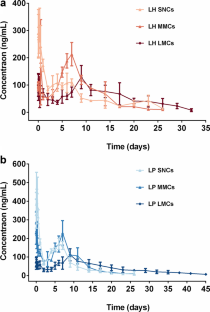
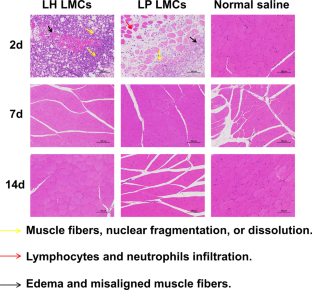
Latuda® is an oral tablet approved by the US Food and Drug Administration (FDA) for the treatment of schizophrenia. However, the clinical efficacy of Latuda® is compromised by patient noncompliance due to frequent daily administration, especially for patients experiencing severe schizophrenia, whose medication is often needed for several months to years. Hence, developing a long-acting injectable formulation of lurasidone is urgently needed. Herein, a poorly water-soluble lurasidone pamoate (LP) salt was synthesized via the facile ion pair-based salt formation technology. The solubility of LP was decreased by 233 folds compared with that of lurasidone hydrochloride (LH). Furthermore, suspensions of LH and LP with three different particle sizes, including 400 nm small-sized nanocrystals (SNCs), 4 μm medium-sized microcrystals (MMCs), and 15 μm large-sized microcrystals (LMCs) were prepared and characterized by powder X-ray diffraction (PXRD) and differential scanning calorimetry (DSC). The in vitro release results showed that particle sizes had great effects on the sustained release of LH, where large-sized particles exhibited superior sustained release than the smaller ones. Besides, LP suspensions exhibited better sustained release than LH suspensions at the same size scale. Moreover, the pharmacokinetics showed that LP LMCs produced an extended in vivo intramuscularly injectable profile for up to 45 days, which was 10 days longer than that of the LH LMCs. Our findings demonstrated that particle size had appreciable impacts on drug sustained release and provided valuable knowledge for the rational design of optimized micronized suspensions for long-acting injectables.
Graphical Abstract
This is a preview of subscription content, log in via an institution to check access.
Access this article
Subscribe and save
Springer+ Basic
€32.70 /Month
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
Buy Now
Price includes VAT (France)
Instant access to the full article PDF.
Rent this article via DeepDyve
Similar content being viewed by others

Increase in Dissolution Rate of Zotepine via Nanomilling Process — Impact of Dried Nanocrystalline Suspensions on Bioavailability
Article 14 December 2021

An impact of nanocrystals on dissolution rate of Lercanidipine: Supersaturation and crystallization by addition of solvent to antisolvent
Article Open access 29 June 2021
Formulation and Characterization of Sublingual Tablets of Iloperidone Prepared by Microenvironmental pH Regulated Approach
Article 02 October 2020
Data Availability
The datasets generated and analyzed during the current study are publicly available.
References
- Millan MJ, Andrieux A, Bartzokis G, Cadenhead K, Dazzan P, Fusar-Poli P, et al. Altering the course of schizophrenia: progress and perspectives. Nat Rev Drug Discov. 2016;15(7):485–515. https://doi.org/10.1038/nrd.2016.28. ArticleCASPubMedGoogle Scholar
- Phan SV. Medication adherence in patients with schizophrenia. Int J Psychiatry Med. 2016;51(2):211–9. https://doi.org/10.1177/0091217416636601. ArticlePubMedGoogle Scholar
- Xu J, Wang J, Wimo A, Qiu C. The economic burden of mental disorders in China, 2005–2013: implications for health policy. BMC Psychiatry. 2016;16:137. https://doi.org/10.1186/s12888-016-0839-0. ArticlePubMedPubMed CentralGoogle Scholar
- Meltzer HY, Share DB, Jayathilake K, Salomon RM, Lee MA. Lurasidone Improves Psychopathology and Cognition in Treatment-Resistant Schizophrenia. J Clin Psychopharmacol. 2020;40(3):240–9. https://doi.org/10.1097/JCP.0000000000001205. ArticleCASPubMedPubMed CentralGoogle Scholar
- Nolan SF, Roman MW. Lurasidone (latuda(R)): an atypical antipsychotic. Issues Ment Health Nurs. 2012;33(5):342–3. https://doi.org/10.3109/01612840.2012.669025. ArticlePubMedGoogle Scholar
- Corponi F, Fabbri C, Bitter I, Montgomery S, Vieta E, Kasper S, et al. Novel antipsychotics specificity profile: A clinically oriented review of lurasidone, brexpiprazole, cariprazine and lumateperone. Eur Neuropsychopharmacol. 2019;29(9):971–85. https://doi.org/10.1016/j.euroneuro.2019.06.008. ArticleCASPubMedGoogle Scholar
- Jambhekar SS, Breen PJ. Drug dissolution: significance of physicochemical properties and physiological conditions. Drug Discov Today. 2013;18(23–24):1173–84. https://doi.org/10.1016/j.drudis.2013.08.013. ArticleCASPubMedGoogle Scholar
- Mittapelly N, Rachumallu R, Pandey G, Sharma S, Arya A, Bhatta RS, et al. Investigation of salt formation between memantine and pamoic acid: Its exploitation in nanocrystalline form as long acting injection. Eur J Pharm Biopharm. 2016;101:62–71. https://doi.org/10.1016/j.ejpb.2016.01.003. ArticleCASPubMedGoogle Scholar
- Van Ngo H, Park C, Tran TTD, Nguyen VH, Lee BJ. Mechanistic understanding of salt-induced drug encapsulation in nanosuspension via acid-base neutralization as a nanonization platform technology to enhance dissolution rate of pH-dependent poorly water-soluble drugs. Eur J Pharm Biopharm. 2020;154:8–17. https://doi.org/10.1016/j.ejpb.2020.07.001. ArticleCASPubMedGoogle Scholar
- Serajuddin AT. Salt formation to improve drug solubility. Adv Drug Deliv Rev. 2007;59(7):603–16. https://doi.org/10.1016/j.addr.2007.05.010. ArticleCASPubMedGoogle Scholar
- Bastin RJ, Bowker MJ. Slater BJJOPR. Development Salt Selection and Optimisation Procedures for Pharmaceutical New Chemical Entities. 2000;4(5):427–35. CASGoogle Scholar
- Haynes DA, Jones W, Motherwell WDS. A systematic study of lutidine salts formed with the pharmaceutically acceptable salt-forming agent, pamoic acid. Cryst Eng Comm. 2005;7(87). https://doi.org/10.1039/b507408h
- Park EJ, Amatya S, Kim MS, Park JH, Seol E, Lee H, et al. Long-acting injectable formulations of antipsychotic drugs for the treatment of schizophrenia. Arch Pharm Res. 2013;36(6):651–9. https://doi.org/10.1007/s12272-013-0105-7. ArticleCASPubMedGoogle Scholar
- Morris MT, Tarpada SP. Long-Acting Injectable Paliperidone Palmitate: A Review of Efficacy and Safety. Psychopharmacol Bull. 2017;47(2):42–52. PubMedPubMed CentralGoogle Scholar
- Sharma S, Verma A, Teja BV, Shukla P, Mishra PR. Development of stabilized Paclitaxel nanocrystals: In-vitro and in-vivo efficacy studies. Eur J Pharm Sci. 2015;69:51–60. https://doi.org/10.1016/j.ejps.2014.11.012. ArticleCASPubMedGoogle Scholar
- Kahn RS, Giannopoulou A. The safety, efficacy and tolerability of Abilify Maintena for the treatment of schizophrenia. Expert Rev Neurother. 2015;15(9):969–81. https://doi.org/10.1586/14737175.2015.1070670. ArticleCASPubMedGoogle Scholar
- Lamb YN, Keating GM. Paliperidone Palmitate Intramuscular 3-Monthly Formulation: A Review in Schizophrenia. Drugs. 2016;76(16):1559–66. https://doi.org/10.1007/s40265-016-0645-5. ArticleCASPubMedGoogle Scholar
- Rawat A, Burgess DJ. USP apparatus 4 method for in vitro release testing of protein loaded microspheres. Int J Pharm. 2011;409(1–2):178–84. https://doi.org/10.1016/j.ijpharm.2011.02.057. ArticleCASPubMedGoogle Scholar
- Thorat AA, Suryanarayanan R. Characterization of Phosphate Buffered Saline (PBS) in Frozen State and after Freeze-Drying. Pharm Res. 2019;36(7):98. https://doi.org/10.1007/s11095-019-2619-2. ArticleCASPubMedGoogle Scholar
Acknowledgements
Thank you to scidraw website for assistance with the Graphical abstract.
Funding
This work was financially supported by the Science and Technology Innovation Projects for Young and Middle-aged Talents of Shenyang (RC200406).
Author information
- Shuo Li and Ying He are Both contributed to this work equally.
Authors and Affiliations
- Wuya College of Innovation, Shenyang Pharmaceutical University, Shenyang, Liaoning, 110016, People’s Republic of China Shuo Li, Ying He, Dianjun Sun, Zhaomeng Wang, Jiang Yu, Jianying Ye, Zhonggui He & Yongjun Wang
- Shuo Li











